What is the charge on the iron cations in iron(II) chloride and iron (III) chloride, respectively?
1 Answer

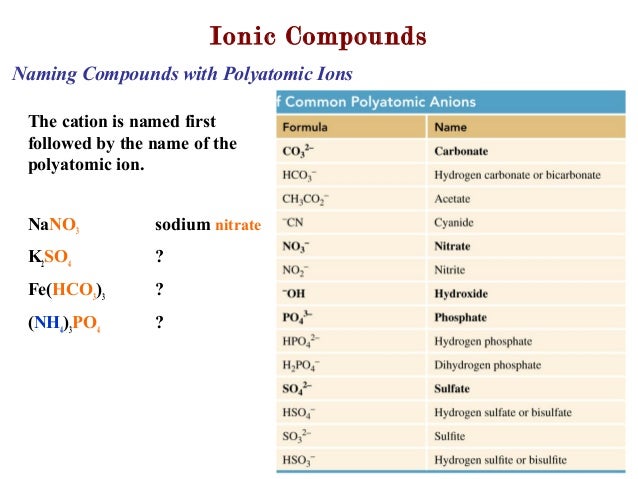
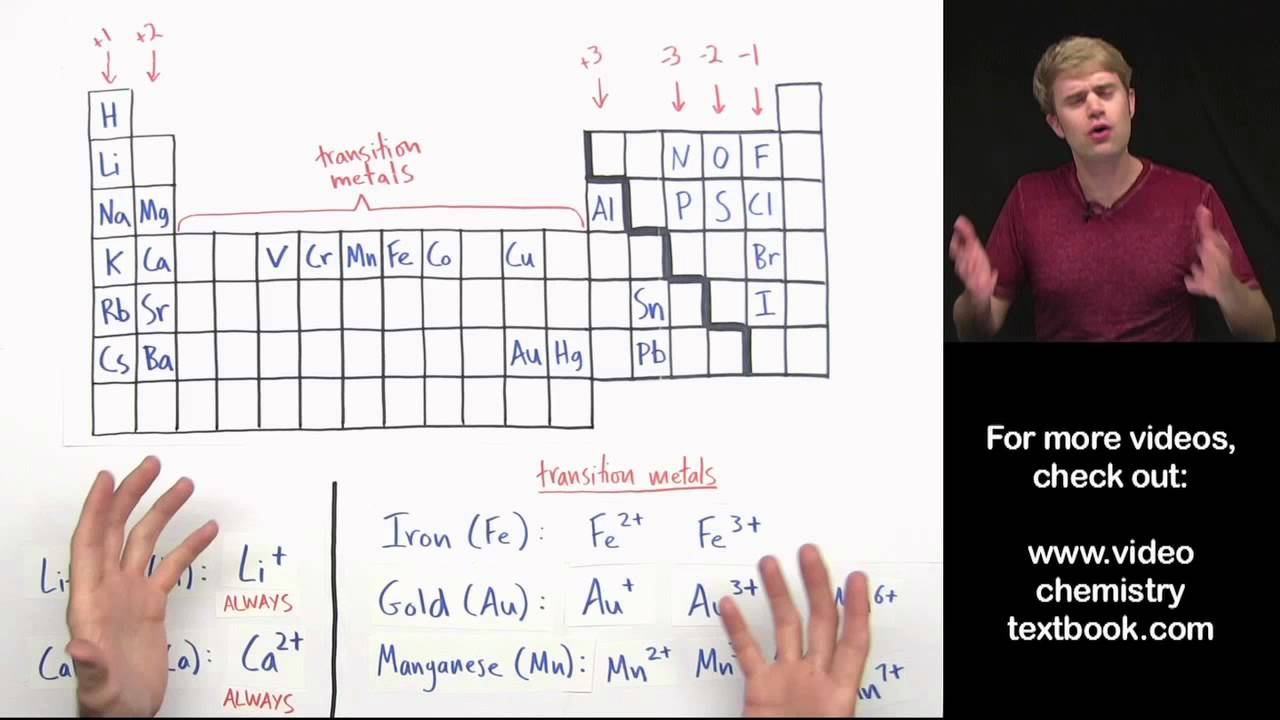
When naming ionic compounds which contain metal ions capable of forming more than one kind of cation, the Roman numeral after the metal's name indicates the charge. Therefore, the iron cation in iron(II) chloride has a charge of 2^+, and the charge on the iron cation in iron(III) chloride has a charge of 3^+'. The Roman numerals after the name of the iron cation indicates the charge on the iron cation. When naming ionic compounds which contain metal ions capable of forming more than one kind of cation, the Roman numeral after the metal's name indicates the charge. Therefore, the iron cation in iron(II) chloride has a charge of 2^+, and the charge on the iron cation in iron(III) chloride has a charge.
The Roman numerals after the name of the iron cation indicates the charge on the iron cation.
Explanation:
When naming ionic compounds which contain metal ions capable of forming more than one kind of cation, the Roman numeral after the metal's name indicates the charge.
Therefore, the iron cation in iron(II) chloride has a charge of #2^+#, and the charge on the iron cation in iron(III) chloride has a charge of #3^+'#.
This video provides some additional examples of how to use Roman numerals when naming compounds.
Element Charges
In the image below, a solution of iron(III) chloride is on the left side and a solution of iron(II) chloride is on the right side.
Related questions
| Arkansas State University Department of Chemistry and Physics |
| Cation/ Anion List |
Fe Cation Charge Table
Students enrolled in Dr. Draganjac's Introduction to Chemistry (CHEM1003), General Chemistry I (CHEM1013) and General Chemistry II (CHEM1023) classes are responsible for learning the names and formulae for the common acids and common reagents and for learning the names, formulae and the charges for the common cations and anions listed below:
+chloride+Cl-+Fe%2B+Cl-+Cl-+Name+the+following%3A.jpg)
When naming ionic compounds which contain metal ions capable of forming more than one kind of cation, the Roman numeral after the metal's name indicates the charge. Therefore, the iron cation in iron(II) chloride has a charge of 2^+, and the charge on the iron cation in iron(III) chloride has a charge of 3^+'. The Roman numerals after the name of the iron cation indicates the charge on the iron cation. When naming ionic compounds which contain metal ions capable of forming more than one kind of cation, the Roman numeral after the metal's name indicates the charge. Therefore, the iron cation in iron(II) chloride has a charge of 2^+, and the charge on the iron cation in iron(III) chloride has a charge.
The Roman numerals after the name of the iron cation indicates the charge on the iron cation.
Explanation:
When naming ionic compounds which contain metal ions capable of forming more than one kind of cation, the Roman numeral after the metal's name indicates the charge.
Therefore, the iron cation in iron(II) chloride has a charge of #2^+#, and the charge on the iron cation in iron(III) chloride has a charge of #3^+'#.
This video provides some additional examples of how to use Roman numerals when naming compounds.
Element Charges
In the image below, a solution of iron(III) chloride is on the left side and a solution of iron(II) chloride is on the right side.
Related questions
| Arkansas State University Department of Chemistry and Physics |
| Cation/ Anion List |
Fe Cation Charge Table
Students enrolled in Dr. Draganjac's Introduction to Chemistry (CHEM1003), General Chemistry I (CHEM1013) and General Chemistry II (CHEM1023) classes are responsible for learning the names and formulae for the common acids and common reagents and for learning the names, formulae and the charges for the common cations and anions listed below:
| Name | Formula | Other name(s) |
| Aluminum | Al+3 | |
| Ammonium | NH4+ | |
| Barium | Ba+2 | |
| Calcium | Ca+2 | |
| Chromium(II) | Cr+2 | Chromous |
| Chromium(III) | Cr+3 | Chromic |
| Copper(I) | Cu+ | Cuprous |
| Copper(II) | Cu+2 | Cupric |
| Iron(II) | Fe+2 | Ferrous |
| Iron(III) | Fe+3 | Ferric |
| Hydrogen | H+ | |
| Hydronium | H3O+ | |
| Lead(II) | Pb+2 | |
| Lithium | Li+ | |
| Magnesium | Mg+2 | |
| Manganese(II) | Mn+2 | Manganous |
| Manganese(III) | Mn+3 | Manganic |
| Mercury(I) | Hg2+2 | Mercurous |
| Mercury(II) | Hg+2 | Mercuric |
| Nitronium | NO2+ | |
| Potassium | K+ | |
| Silver | Ag+ | |
| Sodium | Na+ | |
| Strontium | Sr+2 | |
| Tin(II) | Sn+2 | Stannous |
| Tin(IV) | Sn+4 | Stannic |
| Zinc | Zn+2 |
Fe Cation Charge Formula
Common Anions: (Carbon Ion Charge
ions grouped by charge) (anions grouped by periodic position)| Simple ions: | |||
| Hydride | H- | Oxide | O2- |
| Fluoride | F- | Sulfide | S2- |
| Chloride | Cl- | Nitride | N3- |
| Bromide | Br- | ||
| Iodide | I- | ||
| Oxoanions: | |||
| Arsenate | AsO43- | Phosphate | PO43- |
| Arsenite | AsO33- | Hydrogen phosphate | HPO42- |
| Dihydrogen phosphate | H2PO4- | ||
| Sulfate | SO42- | Nitrate | NO3- |
| Hydrogen sulfate | HSO4- | Nitrite | NO2- |
| Thiosulfate | S2O32- | ||
| Sulfite | SO32- | ||
| Perchlorate | ClO4- | Iodate | IO3- |
| Chlorate | ClO3- | Bromate | BrO3- |
| Chlorite | ClO2- | ||
| Hypochlorite | OCl- | Hypobromite | OBr- |
| Carbonate | CO32- | Chromate | CrO42- |
| Hydrogen carbonate or Bicarbonate | HCO3- | Dichromate | Cr2O72- |
| Anions from Organic Acids: | |||
| Acetate | CH3COO- | formate | HCOO- |
| Others: | |||
| Cyanide | CN- | Amide | NH2- |
| Cyanate | OCN- | Peroxide | O22- |
| Thiocyanate | SCN- | Oxalate | C2O42- |
| Hydroxide | OH- | Permanganate | MnO4- |
